The FDA serves as the primary regulatory authority for breast implants in the United States, overseeing their safety, effectiveness, and availability. This federal agency's role extends far beyond simple approval processes – they monitor ongoing safety data, investigate adverse events, and mandate manufacturer studies to track long-term outcomes. Their breast implant section has become an essential resource for patients seeking unbiased, science-based information free from marketing influence.
The agency's approach to breast implant information reflects their regulatory mandate. Rather than promoting procedures, they focus on risk communication and informed consent. Their materials explain the approval process for different implant types, including the rigorous testing required before market authorization. Historical context about silicone implant moratoriums and subsequent re-approvals helps readers understand the evolution of safety standards.
One standout feature is their comprehensive adverse event reporting system. Patients and healthcare providers can report complications directly to the FDA, contributing to ongoing safety surveillance. The agency publishes regular safety communications when new risks emerge or when data analysis reveals concerning trends. This real-time monitoring has led to important safety updates, including black box warnings about BIA-ALCL risks and requirements for patient decision checklists.
The FDA's breast implant registry represents a significant step toward long-term safety monitoring. They're working with manufacturers and healthcare facilities to track implant performance over time, gathering data that will inform future safety recommendations. This systematic approach to post-market surveillance goes beyond what any single manufacturer or medical society could accomplish independently.
Educational materials from the FDA take a notably different tone than those from industry sources. They emphasize that breast implants aren't lifetime devices and that most patients will need additional surgeries. Complication rates, rupture statistics, and revision surgery frequencies are presented with scientific precision. The agency also addresses emerging concerns about systemic symptoms some patients attribute to their implants, acknowledging ongoing research while avoiding premature conclusions.
The regulatory framework section explains how different implant types receive approval and what studies manufacturers must conduct. Premarket approval applications, clinical trial requirements, and post-approval studies are described in accessible language. This transparency helps patients understand why certain implants are available in other countries but not the United States, and what safety data supports approved products.
FDA's collaboration with international regulatory agencies brings global perspective to breast implant safety. They share data with counterparts in Europe, Canada, and Australia, coordinating responses to emerging safety signals. This international cooperation has strengthened safety standards worldwide and led to harmonized approaches to risk communication.
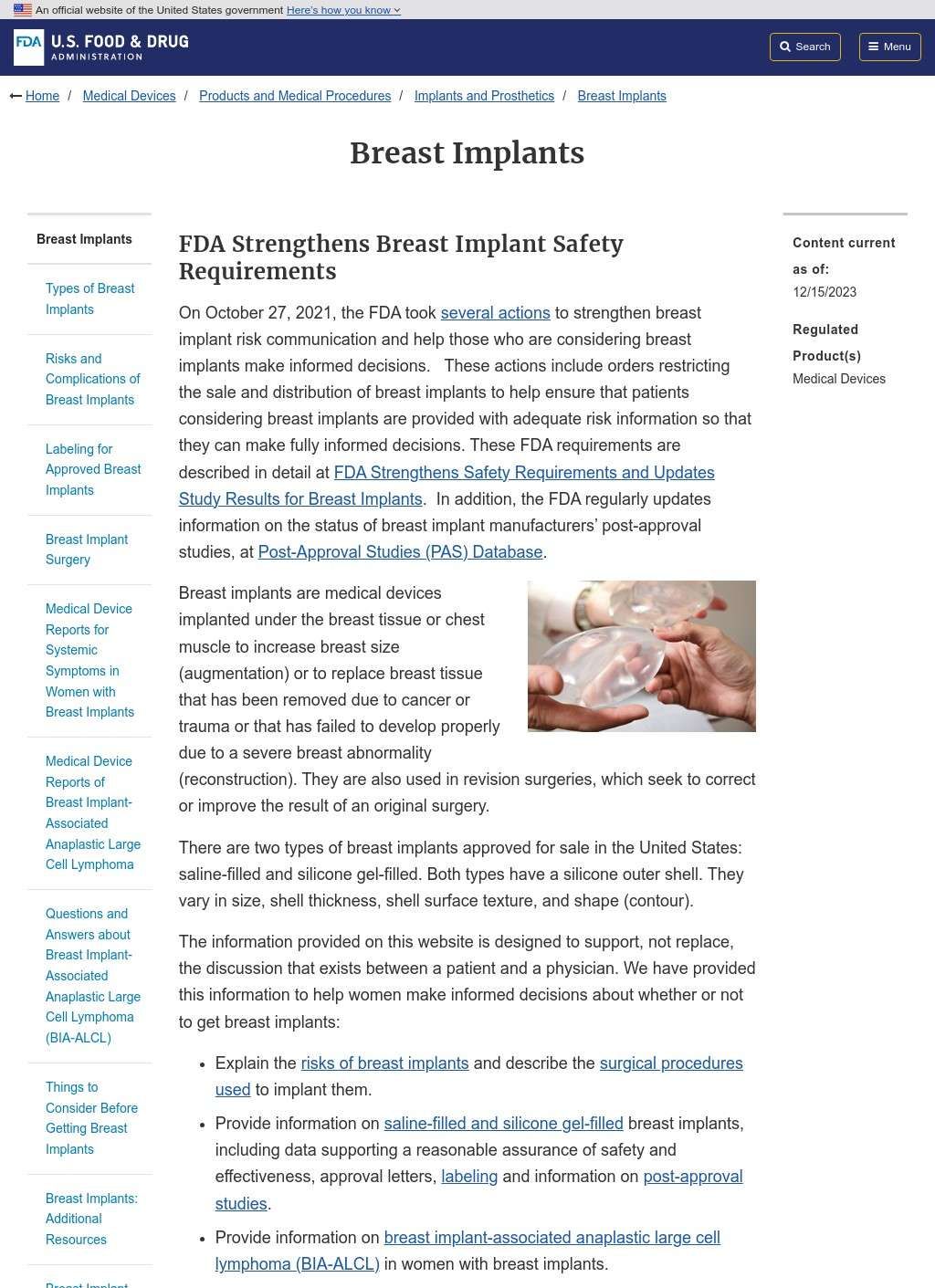
The website features detailed profiles of each approved implant model, including indications, contraindications, and specific warnings. These profiles go beyond marketing materials to include clinical trial data, revision rates, and known complications. Patients can compare different options using objective criteria rather than relying solely on surgeon recommendations or manufacturer claims.
Recent additions to FDA guidance include strengthened informed consent requirements. They now mandate that surgeons provide patients with standardized information about risks and ensure patients have adequate time to review materials before surgery. This patient-centered approach aims to reduce decision regret and improve satisfaction with surgical outcomes.
The agency maintains multiple communication channels for public engagement. Email updates alert subscribers to new safety information, public meetings allow stakeholder input on policy decisions, and citizen petitions provide a formal mechanism for requesting regulatory action. Their commitment to transparency includes publishing meeting transcripts, advisory committee recommendations, and internal safety assessments.
Contact with the FDA for breast implant concerns happens through several channels. Their MedWatch system accepts adverse event reports online or by phone, with trained staff available to assist with submissions. General inquiries go through their consumer affairs branch, which can provide information about specific products or connect callers with appropriate resources. Response times vary, but the agency prioritizes safety-related communications.